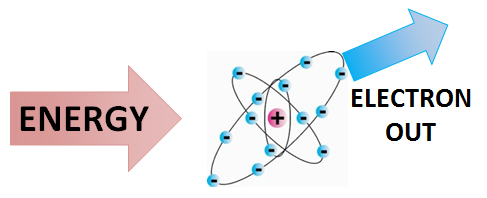
What Happens When an Electron Is Removed From an Atom
What happens if two atoms of equal electronegativity bond together. Hence while dealing with large amount of charges.
A bird perches on a bare high power line and nothing happens.

. This happens because elements further down the periodic table have more electron shells which increases the base size of the atom. When an electric field E is applied across a piece of material the electrons respond by moving with an average velocity called the drift velocity. In practice the charges on bodies are large whereas the charge on electrons are smaller.
Fluorine the most electronegative element is assigned a value of 40 and values range down to caesium and francium which are the least electronegative at 07. The Townsend discharge is a good example of the creation of positive ions and free electrons due to ion impact. The increase in principle energy levels outweighs the increased nuclear charge of the atom hence the atomic radius.
What happens to the temperature of a block of ice when you put a Bunsen burner underneath it. It is a cascade reaction involving electrons in a region with a sufficiently high. What happens to an element Z if its atom gains 3 electrons.
Question_answer57 In the atom of an element X 6 electrons are present in the outermost shell. Every higher principal energy level has orbitals that are bigger than the orbitals found in the smaller energy levels. What would be the nature and value of charge on the ion formed if this electron is removed from the outermost shell.
A man standing on the ground touches the same line. You might think that the temperature goes up smoothly but thats not what happens. Lets look at the heating curve for water.
Electron mobility is almost always specified in units of cm 2 V. The graph of temperature against time is called a heating curve. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons.
Adiabatic ionization is a form of ionization in which an electron is removed from or added to an atom or molecule in its lowest energy state to form an ion in its lowest energy state. The Pauling scale is the most commonly used. Electron and hole mobility are special cases of electrical mobility of charged particles in a fluid under an applied electric field.
If it acquires noble gas configuration by accepting. Then the electron mobility μ is defined as. If electron of charge e is added or removed from a charged body there is not much change on the charge of the body.

Atomic Structure Nucleus Proton Neutron Electron Mass Charge Isotopes Electron Arrangement Rutherford Bohr Model Of Atom Allotropes History Of Atomic Structure Model Development Ionisation Ions Gcse Chemistry Revision Notes Quizzes Ks4 Science
What Happens If You Removed All The Electrons From An Atom Quora
What Happens If You Removed All The Electrons From An Atom Quora
No comments for "What Happens When an Electron Is Removed From an Atom"
Post a Comment